What Is the Unique Characterization of a Ph Buffer
Since we are doing Bio-similiar drugs so we dont have any old data for pH reference. Place the vial with buffer solution pH 401 on the magnetic stirrer.
DM 1 week 2.

. In this pH range each buffer species is either neutral or negatively charged. Gel filtration showed that ACTS is a tetramer in sodium phosphate buffer. However the development of a GLP-1-based pharmaceutical agent ha.
The compatibility of drug with polymer was confirmed by Fourier transform infrared spectroscopy FT-IR. Hybridization of radiolabelled cDNA probes prepared using total RNA from bacteria exposed to buffer at either. All the formulation were subjected to percentage yield drug entrapment efficiency drug content particle size swelling study In phosphate buffer pH 12 as well as in phosphate buffer pH 74 and in vitro drug release study.
To study the effect of mannitol or trehalose on the crystallization behavior of solutes in phosphate buffered saline PBS when the solutions were frozen and freeze-dried. A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. Its pH value doesnt really alt.
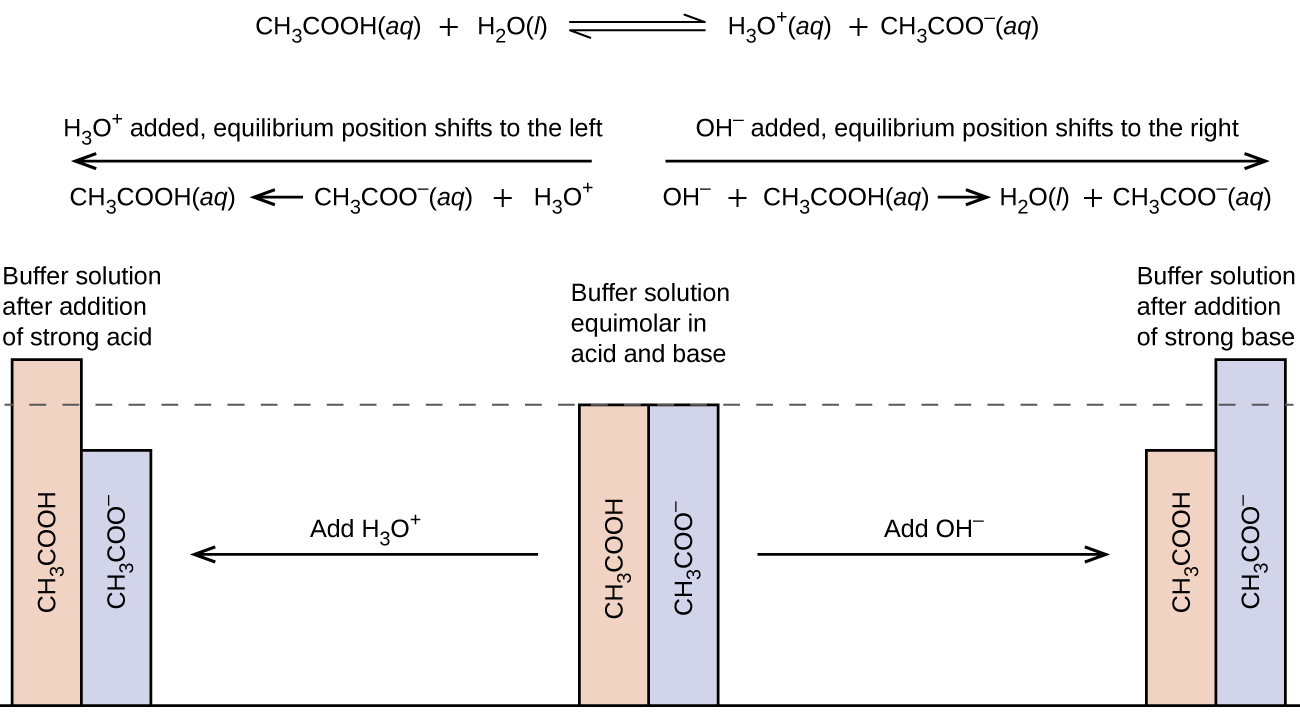
PH is a measure of the hydrogen ion concentration in an aqueous solution. They can be made in three ways. It can be important to maintain a certain pH so we use a buffer solution.
05 and P 0. See full answer below. In Triscitrate buffer the absorbance A520 P 0.
An aqueous solution of a WEAK acid HA and its conjugate base A-. PH Gradient Buffer Concentrates The building blocks of the pH gradient platform are two multicomponent zwitterionic buffer concentrates prepared using a patent pending formulation. Thoroughly rinse the electrode with distilled water and gently dry with a piece of paper towel.
The kinetic investigations resulted in a K M value of 1205 45 mM for maltose and a K M value of 3431 138 mM for trehalose. Immerse the glass electrode in the buffer solution the. A pH of buffered GLA-AFs at the following time points.
For PCprocess characterization purpose we need 3 specific pH for 5mM His buffer. PH of a buffer solution depends on. Its pH changes very little when a small amount of strong acid or base is added to it.
Pylori genes responsive to low pH the authors assembled a high-density array of PCR-amplified random genomic DNA. The pKa of the acid and the ratio A- HA. Buffer salts pH and ionic strength all impact stability and yield and can decrease AAV infectivity.
- buffer solution pH 401 - buffer solution pH 700 Mount the combined glass electrode in the holder. PH pKa log A- HA This equation is used to determine the pH of a buffer solution. Buffer two buffers borate and phosphate having different pH 65 75 and 85 and same molarity 01 M were evaluated with a purpose to determine the suitable buffer required for.
Buffer A is titrated to pH 56 and Buffer B is titrated to pH 102. Calibrate your pH electrode with standard buffers at pH 40 usually a pink solution 70 usually a yellow solution and pH 100 usually a. In this pH range each buffer species is either neutral or negatively charged.
Buffer A is titrated to pH 56 and Buffer B is titrated to pH 102. Therefore they will not be retained by the cation exchange column stationary phase and serve as good buffers for the mobile phase and the stationary phase. A unique characteristic of pH buffer is that it maintains its pH level regardless of whether you add acids or bases to it.
001 and correlation coefficient changed with incubation time and λmax shifts. For a given buffer solution there is a working pH range and a set amount of acid or base that can be neutralized before the pH will change. Proper buffer selection and stability characterization are critical to deter-mine the best conditions but most workflows are a hassle due to high sample volume requirements and time-consuming buffer exchange.
1 See answer Advertisement Advertisement baileyann4389 is waiting for your help. Operating the pH meter For a pH electrode to work correctly the filling hole must be open. The unique characteristic of a pH buffer is that It has a specific pH level and is resistant to any pH changes.
The enzyme was metal ion independent and exhibited maximal activity in sodium phosphate buffer pH 75 at 30 C. Buffers are used to maintain a stable pH in a solution as they can neutralize small quantities of additional acid of base. Prepared using a patent pending formulation.
A buffer may also be called a pH buffer hydrogen ion buffer or buffer solution. Add your answer and earn points. In other words it will.
Glucagon-like peptide-1-7-36 GLP-1 is a hormone derived from the proglucagon molecule which is considered a highly desirable antidiabetic agent mainly due to its unique glucose-dependent stimulation of insulin secretion profiles. Characterization of pH and size stability of GLA-AF with various buffer compositions. View the full answer.
Baileyann4389 baileyann4389 1 week ago Chemistry High School answered What is the unique characterization of a ph buffer. Find an answer to your question what is the unique characterization of a ph buffer. PBS pH 75 at RT either alone or with trehalose 5 wv or mannitol 1 wv were frozen and characterized using low temperature differential scanning calorimetry DSC X-ray.
Check that it is prior to calibrating. Helicobacter pylori is unique among bacterial pathogens in its ability to persist in the acidic environment of the human stomach.

Buffer Solution Its Characteristics Types And Preparations

Xtractor Buffer Is Optimized For Superior Protein Yield Optimization Buffer Biological Activity
No comments for "What Is the Unique Characterization of a Ph Buffer"
Post a Comment